Feasibility:
When beginning with a concept drug candidate, the first important step in an efficient drug development plan is to determine its feasibility. A properly performed strategic assessment determines the key components of the integrated product development plan.



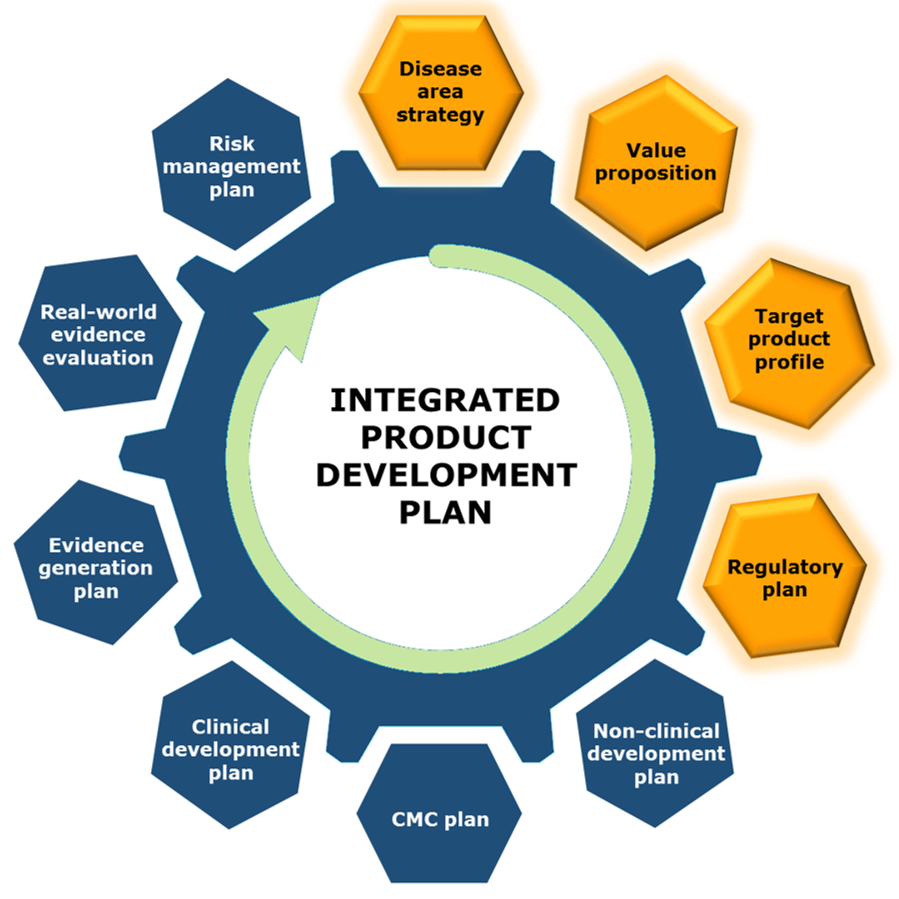
Integrated Product Development Plan
Without a well-developed development plan, it is possible to reach a desired goal. But with an informed plan, it is possible to reach a desired destination by planning for efficiency from the beginning, spending the least amount of time and money to get there.
At Praxis Scientific, we believe that a strategic assessment is the first key to a drug candidate's success, making go/no-go informed decisions at the beginning, and evaluating and planning for scientific, medical, regulatory, and commercial aspects of a product's development.
WE ARE ACCEPTING NEW PROJECTS
Contact us to find out more about our drug development services and how we can help you throughtout all stages of your project - from product ideation to Phase IV.
